Modern Chemistry Workbook Study Guide Answer Key
- Modern Chemistry Review Answers
- Modern Chemistry Review Answer Key
- Modern Chemistry Holt Textbook Answers
DOWNLOAD HOLT MODERN CHEMISTRY STUDY GUIDE ANSWER KEY holt modern chemistry study pdf Management Tips. All chemistry teachers should start the year by having students and parents/guardians.
1 Modern Chemistry Study Guide 2 CAPTER 1 REVIEW Matter and Change SECTION 1 SORT ANSWER Answer the following questions in the space provided. Technological development of a chemical product often (a) lags behind basic research on the same substance. (b) does not involve chance discoveries.
(c) is driven by curiosity. (d) is done for the sake of learning something new. The primary motivation behind basic research is to (a) develop new products. (b) make money. (c) understand an environmental problem. (d) gain knowledge.
Applied research is designed to (a) solve a particular problem. (b) satisfy curiosity. (c) gain knowledge. (d) learn for the sake of learning. Chemistry is usually classified as (a) a biological science. (b) a physical science. (c) a social science.
(d) a computer science. Define the six major branches of chemistry. MODERN CEMISTRY MATTER AND CANGE 1 3 SECTION 1 continued 6. For each of the following types of chemical investigations, determine whether the investigation is basic research, applied research, or technological development.
More than one choice may apply. A laboratory in a major university surveys all the reactions involving bromine. A pharmaceutical company explores a disease in order to produce a better medicine. A scientist investigates the cause of the ozone hole to find a way to stop the loss of the ozone layer. A pharmaceutical company discovers a more efficient method of producing a drug.
A chemical company develops a new biodegradable plastic. A laboratory explores the use of ozone to inactivate bacteria in a drinking-water system. Give examples of two different instruments routinely used in chemistry.
What are microstructures? What is a chemical? What is chemistry? 2 MATTER AND CANGE MODERN CEMISTRY 4 CAPTER 1 REVIEW Matter and Change SECTION 2 SORT ANSWER Answer the following questions in the space provided. Classify each of the following as a homogeneous or heterogeneous substance. Energy drink e. Oil-and-vinegar salad dressing f.
Classify each of the following as a physical or chemical change. Ice melting b. Paper burning c. Metal rusting d. Gas pressure increasing e. Liquid evaporating f.
Food digesting 3. Compare a physical change with a chemical change. MODERN CEMISTRY MATTER AND CANGE 3 5 SECTION 2 continued 4. Compare and contrast each of the following terms: a. Mass and matter b.
Atom and compound c. Physical property and chemical property d. Homogeneous mixture and heterogeneous mixture 5. Using circles to represent particles, draw a diagram that compares the arrangement of particles in the solid, liquid, and gas states.
Solid Liquid Gas 6. Ow is energy involved in chemical and physical changes?
4 MATTER AND CANGE MODERN CEMISTRY 6 CAPTER 1 REVIEW Matter and Change SECTION 3 SORT ANSWER Answer the following questions in the space provided. A horizontal row of elements in the periodic table is called a(n).
The symbol for the element in Period 2, Group 13, is. Elements that are good conductors of heat and electricity are. Elements that are poor conductors of heat and electricity are. A vertical column of elements in the periodic table is called a(n). The ability of a substance to be hammered or rolled into thin sheets is called. Is an element that is soft and easy to cut cleanly with a knife likely to be a metal or a nonmetal? The elements in Group 18, which are generally unreactive, are called.
At room temperature, most metals are. Name three characteristics of most nonmetals. Name three characteristics of metals. Name three characteristics of most metalloids. Name two characteristics of noble gases. MODERN CEMISTRY MATTER AND CANGE 5 7 SECTION 3 continued 14. What do elements of the same group in the periodic table have in common?
Within the same period of the periodic table, how do the properties of elements close to each other compare with the properties of elements far from each other? You are trying to manufacture a new material, but you would like to replace one of the elements in your new substance with another element that has similar chemical properties. Ow would you use the periodic table to choose a likely substitute?
What is the difference between a family of elements and elements in the same period? Complete the table below by filling in the spaces with correct names or symbols.
Name of element Symbol of element Aluminum Ca Mn Nickel Potassium Cobalt Ag 6 MATTER AND CANGE MODERN CEMISTRY 8 CAPTER 1 REVIEW Matter and Change MIXED REVIEW SORT ANSWER Answer the following questions in the space provided. Classify each of the following as a homogeneous or heterogeneous substance. Plastic wrap b. Iron filings e. Cement sidewalk c.
Granola bar 2. For each type of investigation, select the most appropriate branch of chemistry from the following choices: organic chemistry, analytical chemistry, biochemistry, theoretical chemistry. More than one branch may be appropriate. A forensic scientist uses chemistry to find information at the scene of a crime. A scientist uses a computer model to see how an enzyme will function.
A professor explores the reactions that take place in a human liver. An oil company scientist tries to design a better gasoline. An anthropologist tries to find out the nature of a substance in a mummy s wrap. A pharmaceutical company examines the protein on the coating+ of a virus. For each of the following types of chemical investigations, determine whether the investigation is basic research, applied research, or technological development. More than one choice may apply.
A university plans to map all the genes on human chromosomes. A research team intends to find out why a lake remains polluted to try to find a way to clean it up. A science teacher looks for a solvent that will allow graffiti to be removed easily. A cancer research institute explores the chemistry of the cell.
A professor explores the toxic compounds in marine animals. MODERN CEMISTRY MATTER AND CANGE 7 9 MIXED REVIEW continued 4. Use the periodic table to identify the name, group number, and period number of the following elements: a. What is the difference between extensive and intensive properties?
Consider the burning of gasoline and the evaporation of gasoline. Which process represents a chemical change and which represents a physical change? Explain your answer. Describe the difference between a heterogeneous mixture and a homogeneous mixture, and give an example of each. Construct a concept map that includes the following terms: atom, element, compound, pure substance, mixture, homogeneous, and heterogeneous. 8 MATTER AND CANGE MODERN CEMISTRY 10 CAPTER 2 REVIEW Measurements and Calculations SECTION 1 SORT ANSWER Answer the following questions in the space provided. Determine whether each of the following is an example of observation and data, a theory, a hypothesis, a control, or a model.
A research team records the rainfall in inches per day in a prescribed area of the rain forest. The square footage of vegetation and relative plant density per square foot are also measured. The intensity, duration, and time of day of the precipitation are noted for each precipitation episode. The types of vegetation in the area are recorded and classified.
The information gathered is compared with the data on the average precipitation and the plant population collected over the last 10 years. The information gathered by the research team indicates that rainfall has decreased significantly. They propose that deforestation is the primary cause of this phenomenon.
When 10.0 g of a white, crystalline sugar are dissolved in 100. Ml of water, the solution is observed to freeze at 0.54 C, not 0.0 C.
The system is denser than pure water. Which parts of these statements represent quantitative information, and which parts represent qualitative information? Compare and contrast a model with a theory.

MODERN CEMISTRY MEASUREMENTS AND CALCULATIONS 9 11 SECTION 1 continued 4. Evaluate the models shown below. Describe how the models resemble the objects they represent and how they differ from the objects they represent. Ow many different variables are represented in the two graphs shown below? Four Temperature ( F) Aug. May June July Rainfall (inches) Jan.
May June July Aug. 10 MEASUREMENTS AND CALCULATIONS MODERN CEMISTRY 12 CAPTER 2 REVIEW Measurements and Calculations SECTION 2 SORT ANSWER Answer the following questions in the space provided. Complete the following conversions: a.
100 ml L b g cg c. 400 cm 3 L d. 400 cm 3 m 3 2. For each measuring device shown below, identify the quantity measured and tell when it would remain constant and when it would vary. MODERN CEMISTRY MEASUREMENTS AND CALCULATIONS 11 13 SECTION 2 continued 3. Use the data found in Table 4 on page 38 of the text to answer the following questions: a. If ice were denser than liquid water at 0 C, would it float or sink in water?
Modern Chemistry Review Answers
Water and kerosene do not dissolve readily in one another. If the two are mixed, they quickly separate into layers.
Which liquid floats on top? The other liquids in Table 4 that do not dissolve in water are gasoline, turpentine, and mercury. Which of these liquids would settle to the bottom when mixed with water? Use the graph of the density of aluminum below to determine the approximate mass of aluminum samples with the following volumes.
8.0 ml Mass vs. Volume of Aluminum b ml c ml d ml Mass (g) Volume (ml) PROBLEMS provided.
Write the answer on the line to the left. Show all your work in the space 5. Aluminum has a density of 2.70 g/cm 3. What would be the mass of a sample whose volume is 10.0 cm 3? A certain piece of copper wire is determined to have a mass of 2.00 g per meter. Ow many centimeters of the wire would be needed to provide 0.28 g of copper?
12 MEASUREMENTS AND CALCULATIONS MODERN CEMISTRY 14 CAPTER 2 REVIEW Measurements and Calculations SECTION 3 SORT ANSWER Answer the following questions in the space provided. Report the number of significant figures in each of the following values: a g d.
64 ml b g e cm c J f cm 2. Write the value of the following operations using scientific notation. A b c The following data are given for two variables, A and B: A B a. In the graph provided, plot the data A B b.
Are A and B directly or inversely proportional? Do the data points form a straight line? Which equation fits the relationship shown by the data? A B k (a constant) or A B k (a constant) e.
What is the value of k? MODERN CEMISTRY MEASUREMENTS AND CALCULATIONS 13 15 SECTION 3 continued 4. Carry out the following calculations.
Express each answer to the correct number of significant figures and use the proper units. A m 2.7 m m b ml 2 L 137 ml 300. 547 kpa 346 ml 200 K d K 5.
Round the following measurements to three significant figures. A g b m c L d cm e g PROBLEMS provided. Write the answer on the line to the left. Show all your work in the space 6. A pure solid at a fixed temperature has a constant density. We know that mass density or D m v olume V.
Are mass and volume directly proportional or inversely proportional for a fixed density? If a solid has a density of 4.0 g/cm 3, what volume of the solid has a mass of 24 g? A crime-scene tape has a width of 13.8 cm. A long strip of it is torn off and measured to be 56 m long. Convert 56 m into centimeters. What is the area of this rectangular strip of tape, in cm 2? 14 MEASUREMENTS AND CALCULATIONS MODERN CEMISTRY 16 CAPTER 2 REVIEW Measurements and Calculations MIXED REVIEW SORT ANSWER Answer the following questions in the space provided.
Match the description on the right to the most appropriate quantity on the left. 2 m 3 (a) mass of a small paper clip 0.5 g (b) length of a small paper clip 0.5 kg (c) length of a stretch limousine 600 cm 2 (d) volume of a refrigerator compartment 20 mm (e) surface area of the cover of this workbook (f) mass of a jar of peanut butter 2. A measured quantity is said to have good accuracy if (a) it agrees closely with the accepted value. (b) repeated measurements agree closely. (c) it has a small number of significant figures. (d) all digits in the value are significant. A certain sample with a mass of 4.00 g is found to have a volume of 7.0 ml.
To calculate the density of the sample, a student entered on a calculator. The calculator display shows the answer as a. Is the setup for calculating density correct?
Ow many significant figures should the answer contain? It was shown in the text that in a value such as 4000 g, the precision of the number is uncertain. The zeros may or may not be significant. Suppose that the mass was determined to be 4000 g. Ow many significant figures are present in this measurement? Suppose you are told that the mass lies somewhere between 3950 and 4050 g. Use scientific notation to report the value, showing an appropriate number of significant figures.
If you divide a sample s mass by its density, what are the resulting units? MODERN CEMISTRY MEASUREMENTS AND CALCULATIONS 15 17 MIXED REVIEW continued 6. Three students were asked to determine the volume of a liquid by a method of their choosing. Each performed three trials.
The table below shows the results. The actual volume of the liquid is 24.8 ml. Trial 1 Trial 2 Trial 3 (ml) (ml) (ml) Student A Student B Student C a. Considering the average of all three trials, which student s measurements show the greatest accuracy? Which student s measurements show the greatest precision?
PROBLEMS provided. Write the answer on the line to the left. Show all your work in the space 7. A single atom of platinum has a mass of g. What is the mass of platinum atoms?
A sample thought to be pure lead occupies a volume of 15.0 ml and has a mass of g. Determine its density. Is the sample pure lead? (Refer to Table 4 on page 38 of the text.) c. Determine the percentage error, based on the accepted value for the density of lead. 16 MEASUREMENTS AND CALCULATIONS MODERN CEMISTRY 18 CAPTER 3 REVIEW Atoms: The Building Blocks of Matter SECTION 1 SORT ANSWER Answer the following questions in the space provided.
Why is Democritus s view of matter considered only an idea, while Dalton s view is considered a theory? Give an example of a chemical or physical process that illustrates the law of conservation of mass. State two principles from Dalton s atomic theory that have been revised as new information has become available. The formation of water according to the equation 2 2 O O shows that 2 molecules (made of 4 atoms) of hydrogen and 1 molecule (made of 2 atoms) of oxygen produce 2 molecules of water. The total mass of the product, water, is equal to the sum of the masses of each of the reactants, hydrogen and oxygen.
What parts of Dalton s atomic theory are illustrated by this reaction? What law does this reaction illustrate? MODERN CEMISTRY ATOMS: TE BUILDING BLOCKS OF MATTER 17 19 SECTION 1 continued PROBLEMS provided. Write the answer on the line to the left. Show all your work in the space 5. If 3 g of element C combine with 8 g of element D to form compound CD, how many grams of D are needed to form compound CD 2?

Modern Chemistry Review Answer Key
A sample of baking soda, NaCO 3, always contains 27.37% by mass of sodium, 1.20% of hydrogen, 14.30% of carbon, and 57.14% of oxygen. Which law do these data illustrate? State the law. Nitrogen and oxygen combine to form several compounds, as shown by the following table. Compound Mass of nitrogen that combines with 1 g oxygen (g) NO 1.70 NO NO Calculate the ratio of the masses of nitrogen in each of the following: NO a.
N O 2 NO 4 NO c. Which law do these data illustrate?
18 ATOMS: TE BUILDING BLOCKS OF MATTER MODERN CEMISTRY 20 CAPTER 3 REVIEW Atoms: The Building Blocks of Matter SECTION 2 SORT ANSWER Answer the following questions in the space provided. In cathode-ray tubes, the cathode ray is emitted from the negative electrode, which is called the. The smallest unit of an element that can exist either alone or in molecules containing the same or different elements is the.
A positively charged particle found in the nucleus is called a(n). A nuclear particle that has no electrical charge is called a(n).
The subatomic particles that are least massive and most massive, respectively, are the and. A cathode ray produced in a gas-filled tube is deflected by a magnetic field. A wire carrying an electric current can be pulled by a magnetic field. A cathode ray is deflected away from a negatively charged object. What property of the cathode ray is shown by these phenomena? Ow would the electrons produced in a cathode-ray tube filled with neon gas compare with the electrons produced in a cathode-ray tube filled with chlorine gas?
Is an atom positively charged, negatively charged, or neutral? Explain how an atom can exist in this state. MODERN CEMISTRY ATOMS: TE BUILDING BLOCKS OF MATTER 19 21 SECTION 2 continued 9. Below are illustrations of two scientists conceptions of the atom. Label the electrons in both illustrations with a sign and the nucleus in the illustration to the right with a sign. On the lines below the figures, identify which illustration was believed to be correct before Rutherford s goldfoil experiment and which was believed to be correct after Rutherford s gold-foil experiment. In the space provided, describe the locations of the subatomic particles in the labeled model of an atom of nitrogen below, and give the charge and relative mass of each particle.
Electron (a possible location of this particle) 20 ATOMS: TE BUILDING BLOCKS OF MATTER MODERN CEMISTRY 22 CAPTER 3 REVIEW Atoms: The Building Blocks of Matter SECTION 3 SORT ANSWER Answer the following questions in the space provided. Explain the difference between the mass number and the atomic number of a nuclide. Why is it necessary to use the average atomic mass of all isotopes, rather than the mass of the most commonly occurring isotope, when referring to the atomic mass of an element? Ow many particles are in 1 mol of carbon?
1 mol of lithium? 1 mol of eggs? Will 1 mol of each of these substances have the same mass? Explain what happens to each of the following as the atomic masses of the elements in the periodic table increase: a.
Modern Chemistry Holt Textbook Answers
The number of protons b. The number of electrons c. The number of atoms in 1 mol of each element MODERN CEMISTRY ATOMS: TE BUILDING BLOCKS OF MATTER 21 23 SECTION 3 continued 5. Use a periodic table to complete the following chart: Element Symbol Atomic number Mass number Europium-151 Tellurium Ag 6. List the number of protons, neutrons, and electrons found in zinc-66.
Protons neutrons electrons PROBLEMS provided. Write the answer on the line to the left. Show all your work in the space 7. What is the mass in grams of mol of oxygen atoms?
Ow many moles of aluminum exist in g of aluminum? Ow many atoms are in g of magnesium? What is the mass in grams of 100 atoms of the carbon-12 isotope?
22 ATOMS: TE BUILDING BLOCKS OF MATTER MODERN CEMISTRY 24 CAPTER 3 REVIEW Atoms: The Building Blocks of Matter MIXED REVIEW SORT ANSWER Answer the following questions in the space provided. The element boron, B, has an atomic mass of amu according to the periodic table. Owever, no single atom of boron has a mass of exactly amu. Ow can you explain this difference? Ow did the outcome of Rutherford s gold-foil experiment indicate the existence of a nucleus?
Ibuprofen, C O 2, that is manufactured in Michigan contains 75.69% by mass carbon, 8.80% hydrogen, and 15.51% oxygen. If you buy some ibuprofen for a headache while you are on vacation in Germany, how do you know that it has the same percentage composition as the ibuprofen you buy at home? Complete the following chart, using the atomic mass values from the periodic table: Compound Mass of Fe (g) Mass of O (g) Ratio of O:Fe FeO Fe 2 O 3 Fe 3 O 4 MODERN CEMISTRY ATOMS: TE BUILDING BLOCKS OF MATTER 23 25 MIXED REVIEW continued 5. Complete the following table: Atomic Mass Number Number Number Element Symbol number number of protons of neutrons of electrons Sodium 22 F PROBLEMS provided. Write the answer on the line to the left. Show all your work in the space 6.
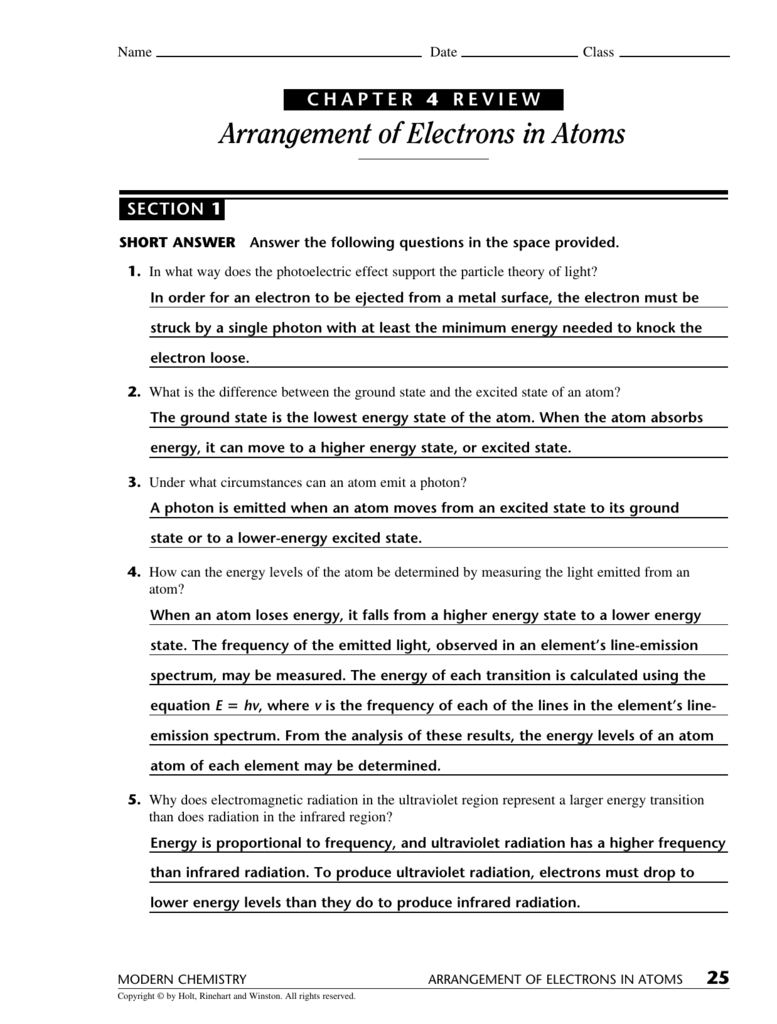
Ow many atoms are there in 2.50 mol of hydrogen? Ow many atoms are there in 2.50 mol of uranium? Ow many moles are present in 107 g of sodium? A certain element exists as three natural isotopes, as shown in the table below. Percent natural Isotope Mass (amu) abundance Mass number Calculate the average atomic mass of this element. 24 ATOMS: TE BUILDING BLOCKS OF MATTER MODERN CEMISTRY 26 CAPTER 4 REVIEW Arrangement of Electrons in Atoms SECTION 1 SORT ANSWER Answer the following questions in the space provided. In what way does the photoelectric effect support the particle theory of light?
What is the difference between the ground state and the excited state of an atom? Under what circumstances can an atom emit a photon? Ow can the energy levels of the atom be determined by measuring the light emitted from an atom? Why does electromagnetic radiation in the ultraviolet region represent a larger energy transition than does radiation in the infrared region?
MODERN CEMISTRY ARRANGEMENT OF ELECTRONS IN ATOMS 25 27 SECTION 1 continued 6. Which of the waves shown below has the higher frequency? (The scale is the same for each drawing.) Explain your answer. Wavelength Wavelength Wave A Wave B 7. Ow many different photons of radiation were emitted from excited helium atoms to form the spectrum shown below? Explain your answer. Spectrum for helium PROBLEMS provided.
Write the answer on the line to the left. Show all your work in the space 8.
What is the frequency of light that has a wavelength of 310 nm? What is the wavelength of electromagnetic radiation if its frequency is z? 26 ARRANGEMENT OF ELECTRONS IN ATOMS MODERN CEMISTRY 28 CAPTER 4 REVIEW Arrangement of Electrons in Atoms SECTION 2 SORT ANSWER Answer the following questions in the space provided.
Ow many quantum numbers are used to describe the properties of electrons in atomic orbitals? (a) 1 (c) 3 (b) 2 (d) 4 2. A spherical electron cloud surrounding an atomic nucleus would best represent (a) an s orbital. (c) a combination of two different p orbitals. (b) a p orbital. (d) a combination of an s and a p orbital. Ow many electrons can an energy level of n 4 hold?
(a) 32 (c) 8 (b) 24 (d) 6 4. Ow many electrons can an energy level of n 2 hold? (a) 32 (c) 8 (b) 24 (d) 6 5. Compared with an electron for which n 2, an electron for which n 4 has more (a) spin.
(b) particle nature. (d) wave nature. According to Bohr, which is the point in the figure below where electrons cannot reside? (a) point A (c) point C (b) point B (d) point D Orbitals Nucleus A B C D 7. According to the quantum theory, point D in the above figure represents (a) the fixed position of an electron. (b) the farthest position from the nucleus that an electron can achieve.
(c) a position where an electron probably exists. (d) a position where an electron cannot exist. MODERN CEMISTRY ARRANGEMENT OF ELECTRONS IN ATOMS 27 29 SECTION 2 continued 8. Ow did de Broglie conclude that electrons have a wave nature? Identify each of the four quantum numbers and the properties to which they refer. Ow did the eisenberg uncertainty principle contribute to the idea that electrons occupy clouds, or orbitals? Complete the following table: Principal quantum number, n Number of sublevels Types of orbitals ARRANGEMENT OF ELECTRONS IN ATOMS MODERN CEMISTRY 30 CAPTER 4 REVIEW Arrangement of Electrons in Atoms SECTION 3 SORT ANSWER Answer the following questions in the space provided.
State the Pauli exclusion principle, and use it to explain why electrons in the same orbital must have opposite spin states. Explain the conditions under which the following orbital notation for helium is possible: 1s 2s Write the ground-state electron configuration and orbital notation for each of the following atoms: 3. Phosphorus 4.
Potassium MODERN CEMISTRY ARRANGEMENT OF ELECTRONS IN ATOMS 29 31 SECTION 3 continued 6. Which guideline, und s rule or the Pauli exclusion principle, is violated in the following orbital diagrams? 1s 2 2s 2 2p 6 3s 2 a. 1s 2 2s 2 2p 4 b. 30 ARRANGEMENT OF ELECTRONS IN ATOMS MODERN CEMISTRY 32 CAPTER 4 REVIEW Arrangement of Electrons in Atoms MIXED REVIEW SORT ANSWER Answer the following questions in the space provided. Under what conditions is a photon emitted from an atom?
What do quantum numbers describe? What is the relationship between the principal quantum number and the electron configuration? In what way does the figure above illustrate und s rule? In what way does the figure above illustrate the Pauli exclusion principle? MODERN CEMISTRY ARRANGEMENT OF ELECTRONS IN ATOMS 31 33 MIXED REVIEW continued 6. Elements of the fourth and higher main-energy levels do not seem to follow the normal sequence for filling orbitals.
Why is this so? Ow do electrons create the colors in a line-emission spectrum? Write the ground-state electron configuration of the following atoms: a. Copper PROBLEMS provided.
Write the answer on the line to the left. Show all your work in the space 9. What is the wavelength of light that has a frequency of z in a vacuum?
What is the energy of a photon that has a frequency of z? 32 ARRANGEMENT OF ELECTRONS IN ATOMS MODERN CEMISTRY 34 CAPTER 5 REVIEW The Periodic Law SECTION 1 SORT ANSWER Answer the following questions in the space provided.
In the modern periodic table, elements are ordered (a) according to decreasing atomic mass. (b) according to Mendeleev s original design. (c) according to increasing atomic number. (d) based on when they were discovered. Mendeleev noticed that certain similarities in the chemical properties of elements appeared at regular intervals when the elements were arranged in order of increasing (a) density. (c) atomic number. (b) reactivity.
(d) atomic mass. The modern periodic law states that (a) no two electrons with the same spin can be found in the same place in an atom. (b) the physical and chemical properties of an element are functions of its atomic number. (c) electrons exhibit properties of both particles and waves. (d) the chemical properties of elements can be grouped according to periodicity, but physical properties cannot. The discovery of the noble gases changed Mendeleev s periodic table by adding a new (a) period.
The most distinctive property of the noble gases is that they are (a) metallic. (c) metalloid. (b) radioactive. (d) largely unreactive.
Lithium, the first element in Group 1, has an atomic number of 3. The second element in this group has an atomic number of (a) 4. (d) An isotope of fluorine has a mass number of 19 and an atomic number of 9. Ow many protons are in this atom? Ow many neutrons are in this atom?
What is the nuclear symbol of this fluorine atom, including its mass number and atomic number? MODERN CEMISTRY TE PERIODIC LAW 33 35 SECTION 1 continued 8. Samarium, Sm, is a member of the lanthanide series.
Identify the element just below samarium in the periodic table. By how many units do the atomic numbers of these two elements differ? A certain isotope contains 53 protons, 78 neutrons, and 54 electrons. What is its atomic number? What is the mass number of this atom?
What is the name of this element? Identify two other elements that are in the same group as this element. In a modern periodic table, every element is a member of both a horizontal row and a vertical column.
Which one is the group, and which one is the period? Explain the distinction between atomic mass and atomic number of an element.
In the periodic table, the atomic number of I is greater than that of Te, but its atomic mass is less. This phenomenon also occurs with other neighboring elements in the periodic table. Name two of these pairs of elements.
Refer to the periodic table if necessary. 34 TE PERIODIC LAW MODERN CEMISTRY 36 CAPTER 5 REVIEW The Periodic Law SECTION 2 SORT ANSWER provided.